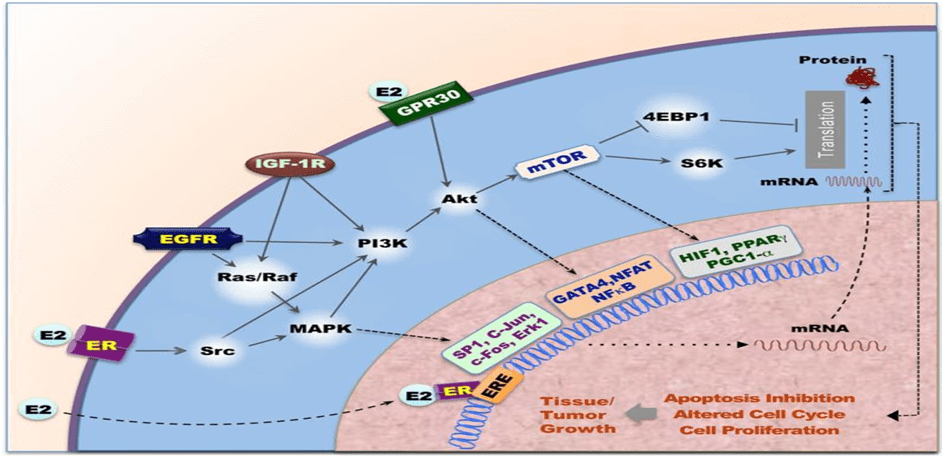
Our cells sends and receive millions of messages in the form of chemical signaling molecules. Cells communicate using these chemical signals. Regardless of nature of the signal, the target cell respond by the means of receptor, which binds the signal molecules(ligand) & initiates a response in target cell. Receptors are chemically protein or glycoprotein molecule which bind to signal molecules(ligand). Binding of ligand to its receptor causes conformational change in receptor that initiates a sequence of reaction leading to specific cellular responses. Based on location, Receptors are being classified as 1) Intracellular Receptors – Cytoplasmic Receptor & Nuclear Receptor 2) Cell Surface Receptors– G protein, Tyrosine Kinase Receptors & Janus Kinase Receptor
Estrogen and estrogen receptors (ERs) are critical regulators of breast epithelial cell proliferation, differentiation, and apoptosis. The Estrogen Receptors(ER) are believed to be a major contributing factor in the malignancy of breast cells. Targeting the ER signaling pathway has been a focal point in the development of breast cancer therapy. Approximately 70% of breast cancers are estrogen receptor-positive and this type of breast cancer is more common in postmenopausal women. Breast cancer, a genetically and clinically heterogeneous disease that originates from the mammary epithelial cells. It remains the leading cause of cancer deaths among females worldwide with about one in eight women (12 %) developing breast cancer in her lifetime. Woman’s risk for breast cancer is linked to her reproductive history and her lifetime hormonal exposure. The levels of estrogen in blood and tissue are associated with breast cancer carcinogenesis. Estrogen receptors belong to the Nuclear Receptor Superfamily. The two different forms of Estrogen Receptors(ER) – ERα and ERβ are coded by two distinct genes ESR1 and ESR2, located on chromosomes 6 and 14, respectively. ERα mediates unregulated cell proliferation in breast cells. However, ERβ opposes the actions of ERα by modulating the expression of ERα-regulated genes and reducing migration of cancer cells. Let’s know about Estrogen signalling pathway and what goes wrong in the pathway resulting in abnormal condition.
ESTROGEN SIGNALLING
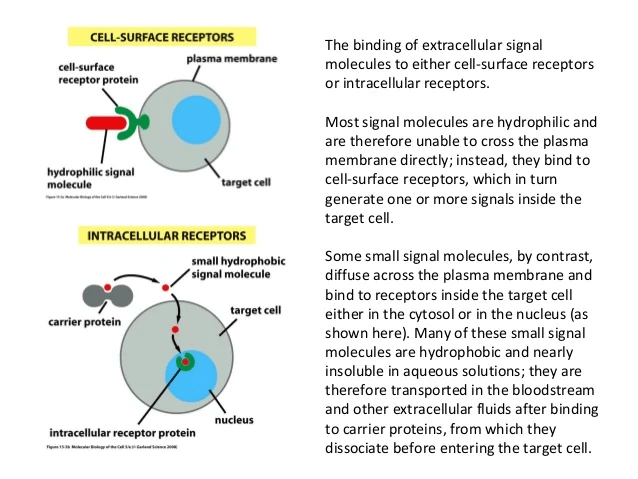
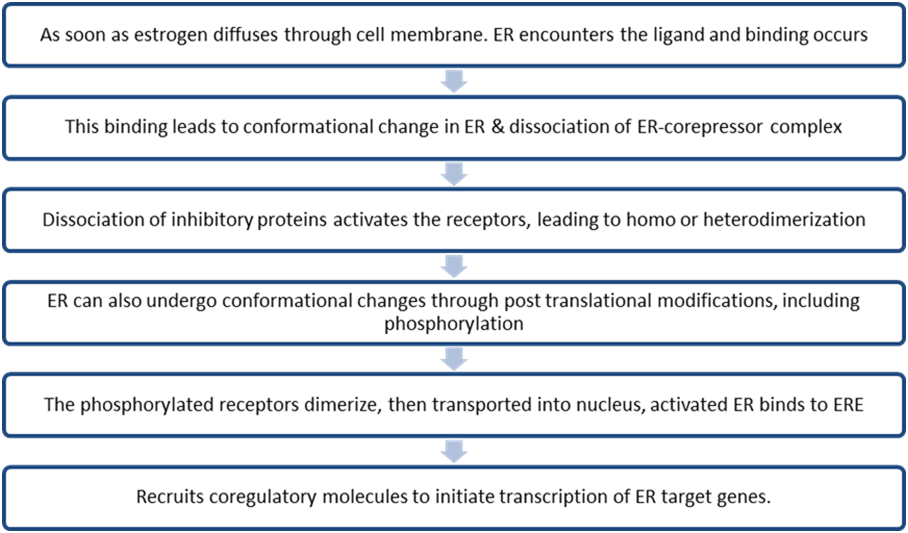
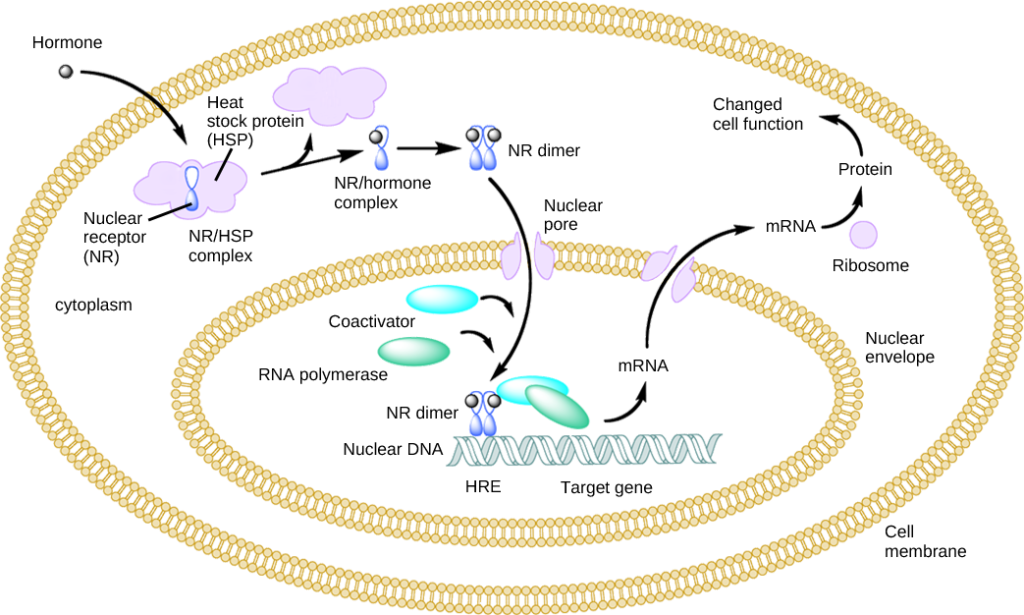
Estrogen signaling includes two distinct pathways often referred to as genomic and non-genomic pathways. ERs are randomly distributed in the cell & are maintained in an inactive state by co-repressor proteins (NCORI, SMRT ). To activate ER- dependent transcription, ER-corepressor complexes need to be dissociated & replaced by co-activator complexes.
GENOMIC PATHWAY
DYSREGULATION OF GENOMIC PATHWAY
Co- activators contain LXXLL motif (L,leucine, X, any A.A) that interacts with ligand binding domain of ERα. These co-regulators are associated with various enzymes such as deacetylase, acetyltransferase, methyltransferase, ATPase, etc which regulate chromatin remodeling & directly or indirectly regulate target gene expression. Deregulation of coregulator expression is associated with tumor progression, metastasis, cancer cell migration & drug resistance. Binding of AIB1 & BCAS3(Breast carcinoma amplified sequence 3) both ERα co-activators are known to induce Breast carcinogenesis.
NON-GENOMIC PATHWAY
Estrogens regulate activity of ERs by serving as ligand, Estrone(E1), Estradiol(E2) &Estriol(E3)- 3 forms of estrogen. E2 is important ligand of ERs. Depending on a cell type, small fraction of ERα is involved in non-genomic action by anchoring to PM. Non genomic signaling of estrogen involves, mobilization of secondary messengers & interaction with membrane receptors such as, IGF-1R(Growth factor-1-receptor), EGFR(Epidermal growth factor receptor), Stimulation of effector molecules such as Src & PI3K, Serine/theronine protein kinase(AKT), MAPK(Mitogen activated protein kinase)
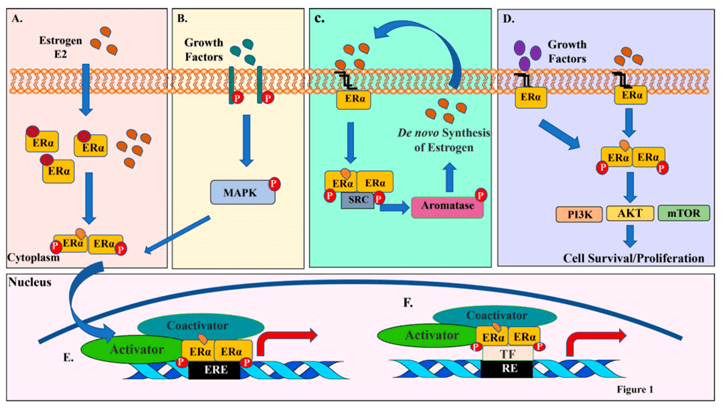
B) Growth factors activate receptor tyrosine kinases, which activate MAPKs. MAPKs can then phosphorylate and activate ERα either independent of E2 or synergize with E2 for optimal ERα activation.
C) Membrane associated ERα interacts and activates SRC kinase upon ligand binding. SRC kinase then phosphorylates and activates aromatase, which catalyzes conversion of androgens to estrogens within cells and amplify both genomic and nongenomic ERα-E2 signaling.
(D) Membrane anchored ERα can also activate various cytoplasmic kinases including PI3K-AKT-mTOR pathway through nongenomic actions, resulting in activation AKT pathway involved in cell proliferation and survival.
| NORMAL REGULATED PATHWAY | DEREGULATED PATHWAY |
| When E2 binds to activated ERα receptor , Depalmitoylation at cysteine477 (detachment of fatty acid molecule from the membrane) The dissociated molecule then interacts with signaling molecule PI3K. PI3K converts PIP2 into PIP3, which interacts with PH domain of AKT Transfer AKT to cell membrane along with PDK1, resulting in conformational change in AKT & exposed T308 & S473 undergoes phosphorylation. Further, PIP3-dependent phosphorylation by m-TOR, resulting in full activation of AKT/Protein Kinase B. Promotes cell Proliferation, survival & apoptosis. | When E2 binds to activated membrane ERα, Palmitoylation at cysteine477 localize ERα to PM. Responsible for ligand-induced activation of PI3K-AKT-mTOR pathway, resulting in Breast cancer cell. |